3.4.5 Compounds Containing the Carbonyl Group - Acylation
Students should:
|
Manufacture of biodiesel
Biofuels are fuels made from recently living organisms. They can be divided into three categories:
- 1 First-generation biofuels are made largely from edible sugars and starches.
- 2 Second-generation biofuels are made from non-edible plant materials.
- 3 Third-generation biofuels are made from algae
and other microbes.
Biodiesel is produced by chemically reacting a fat or oil with an alcohol, in the presence of a catalyst. The product of the reaction is a mixture of methyl esters, which are known as biodiesel, and glycerol, which is a high value co-product. The process is known as transesterification, as shown in the equation in the animations below, where R1, R2, and R3 are long hydrocarbon chains:
There are only five chains that are common in most vegetable oils and animal fats (others are present in small amounts). The relative amounts of the five methyl esters determines the physical properties of the fuel.
Acid chlorides and acid anhydrides
Both of these classes of compounds are very reactive and provide perfect acylating agents (addition of R-CO-). The carbonyl group is susceptible to nucleophilic attack and the chlorine, in the case of acyl halides, makes a very good leaving group.
The reaction mechanism is described as addition followed by elimination.
- 1 The two reagent molecules join together (addition)
- 2 A small molecule is eliminated
In the following reaction schemes ethanoyl chloride is used as the most typical acyl halide and ethanoic anhydride as the most typical anhydride.
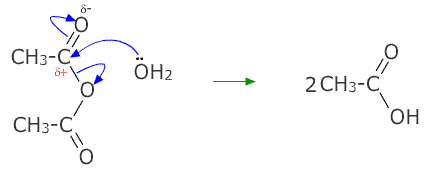
Hydrolysis of ethanoic anhydride
This is the breakdown of compounds using water. Both ethanoyl chloride and ethanoic anhydride are hydrolysed by water. The lone pair on the oxygen attacks at the partially positive carbon of the carbonyl group.
One hydrogen ion is lost from the water molecule and the ethanoic anhydride breaks off an ethanoate ion. The product is two molecules of ethanoic acid.
Hydrolysis of ethanoyl chloride
Ethanoyl chloride is more reactive than ethanoic anhydride and fumes in moist air. The fumes are due to hydrogen chloride gas condensing water droplets forming a mist of hydrochloric acid. The reaction is exothermic.

Reaction of ethanoic anhydride with alcohols and phenols
An alcohol can be considered to be a water molecule in which one of the hydrogen atoms has been replaced by an alkyl group. The mechanism of reaction with acyl halides and anhydrides is the same as that of water.
Ethanoic anhydride

Ethanoyl chloride

Reaction of ammonia with ethanoyl chloride
The lone pair on the nitrogen atom attacks the partially positive carbon of the carbonyl group (it is polarised because of the large electronegativity difference between carbon and oxygen). This attack forces the pi electrons from the carbonyl group onto the oxygen giving it a formal negative charge.
The electron pair on the oxygen then returns into the pi system of the carbonyl group and the chlorine atom leaves as a chloride ion.
At the same time one of the hydrogens is lost from the ammonia group to compensate for the positive charge which had developed there. This can join up with the chloride ion forming hydrogen chloride gas, which leaves the mixture pulling the reaction to the right hand side.
The final result is an amide molecule:

The resultant amide is further stabilised by the lone pair on the nitrogen delocalising into the carbonyl groups pi system. This also helps to drive the reaction to the right hand side.
Anhydrides can carry out very similar reactions, only this time the carboxylate group becomes the leaving group.

The lone pair on the nitrogen of the ammonia molecule initiates the reaction (nucleophilic attack).
Amines also have a lone pair on the nitrogen atom and can react with acyl chlorides and anhydrides in exactly the same way, to produce N-substituted amides.
RCOCl + R'NH2 ![]() RCONHR' + HCl
RCONHR' + HCl
RCOOCOR + R'NH2 ![]() RCONHR' + RCOOH
RCONHR' + RCOOH
Aspirin
Aspirin is probably the most well known of the analgesic (pain relieving) agents. The use of salicylic acid to alleviate pain has been known for thousands of years from the extracts of willow bark. However the acid is too damaging on the mouth and stomach to be taken directly (pKa1 = 2.97).
In 1899, the Bayer Company in Germany patented an ester of salicylic acid, acetylsalicylic acid, and marketed the product as “aspirin”. Their studies showed that this ester was somewhat less of an irritant (pKa = 3.5). It is known today that acetylsalicylic acid is hydrolyzed in the small intestine to salicyclic acid, and is then absorbed into the bloodstream.
Aspirin (acetyl salicylic acid) is the salicylyl ester of ethanoic acid. Salicylic acid is the trivial name for 2-hydroxybenzenecarboxylic acid, a compound that contains both a carboxylic acid group as well as a hydroxy group atached to a benzene ring.
The phenol group is able to esterify with ethanoic anhydride or ethanoyl chloride to produce aspirin:

Aspirin is prepared in the laboratory by simply adding ethanoic anhydride to salicylic acid in the presence of concentrated sulfuric acid and leaving the mixture to react or some 15 minutes. The excess ethanoic anhydride is destroyed by the addition of water. The aspirin can be collected by cooling in ice, filtering and may be purified by recrystallisation from an ethanol/water mix.
Advantage of industrial use of ethanoic anhydride over ethanoyl chloride
In industry, ethanoic anhydride is used rather than ethanoyl chloride for practical purposes. Ethanoyl chloride is very reactive with water and any moisture produces hydrogen chloride gas. This can build up inside containers and pressurise them. This makes ethanoyl chloride irritating and difficult to handle. Besides, ethanoic anhydride is cheaper than ethanoyl chloride (probably the most important industrial consideration).
- 1 Ethanoyl chloride easily broken down by water (hydrolysed)
- 2 Hydrolysis produces toxic and irritant hydrogen chloride fumes
- 3 Ethanoic anhydride is cheaper