3.4.6 Aromatic Chemistry - Bonding
Students should:
|
Benzene
The cyclic hydrocarbon ring structure of benzene forms the basis of aromatic chemistry. There are 6 x sp2 hybridised carbon atoms arranged in a perfect planar hexagon, each attached to a hydrogen atom.
This is usually drawn as a hexagon with a ring inside, or with three alternating double bonds for shorthand representation:
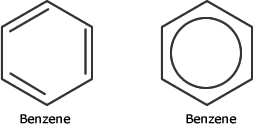
The sp2 hybridisation at each carbon means that only three out of four electrons per carbon is used for sigma bonding, leaving one electron per atom to occupy a delocalised molecular orbital that covers all of the atoms.