3.4.4 Nomenclature and Isomerism in Organic Chemistry - Isomerism
Students should:
|
Isomerism
Isomerism is when two (or more) compounds have the same molecular formula, but differ in some way from one another.
Isomerism can be sub-divided into two different categories.
- Structural (the actual positions of the atoms in the molecule are different relative to one another)
- Stereoisomerism (the molecules are different due to the relative poisitions of the atoms in space)
Structural isomerism
May be positional or functional group isomerism.
Positional isomerism
The molecular formula is the same but the arrangement of atoms in the molecule is different although there is no change in functional group. There may be a difference in the branching of a hydrocarbon, or the position of an adduct to the chain may be different. Structural isomers have different physical and chemical properties.
eg. Methylpropane and butane
In this instance the physical properties show a difference in boiling point with the more branched isomer (the methyl propane) having the lower b.p. The explanation for this is that this molecule is more spherical and has less surface area for the Van der Waal's forces to act.
eg 1-chloropropane and 2-chloropropane
In this case the two isomers will have different reaction rates with sodium hydroxide (nucleophilic substitution by the OH- group). The 2- chloro isomer reacts faster and more easily than the 1- chloro isomer.
Functional group isomerism
The molecular formula is the same but the atoms are arranged in such a way that there is a change in the functional group. This of course produces radical changes in both the physical and chemical properties of the isomers.
|
Example: ethenol and ethanal CH2=CH-OH and CH3CH2OH Example: ethoxyethane and butanol C2H5-O-C2H5 and C4H9OH |
Stereoisomerism
May be sub-divided up into geometric isomerism and optical isomerism
Geometrical isomerism
In this type of isomerism there is a lack of rotation between two asymmetric sides which produces different chemical (and physical) environments. The simplest case is that of an alkene where there is no rotation possible about the double bond (because it would result in rupture of the pi orbital). If there are two different attachments on each side of the double bond then geometrical isomerism results. If the main groups seem to "cross" the double bond then the prefix trans- is used. If they seem to be on the same (lateral) side of the double bond then the prefix cis- is used. In geometrical isomers there are differences in both physical and chemical properties.
|
Example: but - 2 - ene
|
Other situations where rotation is restricted also produce geometric isomerism. This may be due to steric hindrance or rings structures, such as cyclobutane.
E-Z (geometric) isomerism
The EZ system of naming geometric isomers improves on the cis-, trans-, system and allows for identification of all geometric isomers without ambiguity. It is based on the Cahn, Ingold, Prelog system of priorities.
Cahn, Ingold, Prelog priorities
If the two groups with the highest priority are on the same side of the double bond the compound becomes a 'Z' (Zussamen, German = together) and if on opposite sides an 'E' isomer (Entgegen, German = oppposite)
 |
 |
| Z-dichloroethene | E-dichloroethene |
Optical isomerism
When four separate groups are attached to a carbon atom it is possible to have a non-superimposible mirror image of the molecule. This is called optical isomerism.
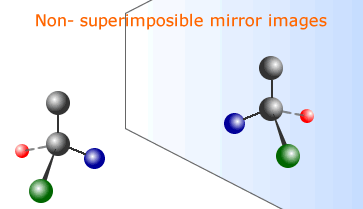
Specific rotation applies to a 1 molar solution at RTP. It is the angle by which a 1 molar solution of an optical isomer rotates the plane of polaried light per cm pathlength.
Optical isomers have identical physical properties (except for polarised light) and identical chemical properties unless reacting with other optical isomers, when the orientation of the two molecules could have a bearing on the final result, for example in biological systems.
Note that this property is used to separate enantiomers to obtain pure optical forms.
Enantiomers
Each of the mirror images of an optically active substance is called an enantiomer.
A solution of an enantiomer (or a pure liquid enantiomer) will rotate the plane of polarised light either to the left (laevorotatory) or to the right (dextrorotatory). The degree of rotation is determined by the nature of the molecule and the concentration of the solution.
Racemates
 An
equimolar solution of two enantiomers has no optical properties as the laevo-rotatory
form counteracts the effects of the dextro-rotatory form. Such a solution is
said to be a racemate or racemic solution.
An
equimolar solution of two enantiomers has no optical properties as the laevo-rotatory
form counteracts the effects of the dextro-rotatory form. Such a solution is
said to be a racemate or racemic solution.
The name comes from the historic separation of racemic acid, so called because it was extracted from grapes (the Latin name for a bunch of grapes was 'racemus').
Racemic acid is an optically inactive mixture of the two enantiomeric forms of tartaric acid, HOOC-CH(OH)-CH(OH)-COOH.
Biological implications of optical isomerism
Living things use enzymes to catalyse biological reactions. These enzmes have binding sites that are optically active and operate ona key-in-door principle. These enzymes can only act on the correct optical isomer, hence most of the molecules in biological systems consist of only one type of enantiomer.
This can have far reaching consequences in the field of pharmacology, unless enough research is carried out during drug testing to ensure that all the optical isomers are non-toxic.
The most publicised tragedy of this kind happened in the 1950's when a tranquiliser drug called thalidomide was released on the market. It soon became apparent that one of the two optical isomers present in the drug has a very damaging effect on the development of the unborn foetus with devastating consequences.
The drug is still on the market today, but with a different tradename and clear warnings that it must not be taken by pregnant women.
