3.4.5 Compounds Containing the Carbonyl Group - Aldehydes and ketones
Students should:
|
Aldehydes and ketones
Both of these groups contain the carbonyl group. They differ in that aldehydes end a carbon chain, while ketones appear in the middle of a carbon chain. This difference gives rise to differing properties, with aldehydes being more reactive and able to behave as reducing agents.
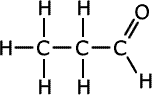 |
 |
| Propanal | Propanone |
The oxidation state of the carbonyl carbon atom in aldehydes and ketones can be calculated in the same way as any oxidation state. The carbonyl group C=O is attached to two other groups, either one alkyl and a hydrogen (all aldehydes except methanal) or two alkyl groups (all ketones).
Using methanal as the example:
(2 x H) + (1 x C) + (1 x O) = 0
(+2) + C + (-2) = 0
Carbon is in the zero oxidation state.
Reactivity of aldehydes and ketones
Aldehydes are reducing agents. This allows them to be oxidised to carboxylic acids. This cannot happen to ketones, as they have no hydrogen atoms attached to the carbonyl carbon.
This forms the basis of tests that distinguish between aldehydes and ketones
Test 1 - Tollens reagent
Tollen's reagent is an ammoniacal solution of silver ions with the formula Ag[NH3]+. Diammine silver(I) ions. These are formed by adding sodium hydroxide solutin dropwise to silver nitrate solution until a light brown precipitate of silver oxide appears. Ammonia solution is then added dropwise until the precipitate re-dissolves.
When an aldehyde is warmed with Tollen's reagent a silver miror forms on the surface of the glassware holding the solution. This is caused by reduction of the silver(I) ions to silver by the aldehyde.
Ketones give no result.
Test 2 - Fehling's solution
This is a dark blue solution of copper ions made by mixing copper sulfate solution (Fehling's A) with potassium sodium tartrate in sodium hydroxide solution (Fehling's B). The mixture produces a complexed copper(II) ion.
The two solutions are mixed together and then heated with a sample of the aldehyde. The appearance of a red precipitate of copper(I) oxide is indicative of aldehyde. Once again the aldehyde is behaving as a reducing agent and changing copper(II) to copper(I).
Ketones give no result
Nucleophilic addition reactions
Both aldehydes and ketones undergo nucleophilic addition with HCN/KCN. The carbon of the carbonyl group is partially positive due to the greater electronegativity of the oxygen. It acts as a source of attraction for nucleophiles, such as the cyanide ion, CN-.

The reaction is the same for ketones, only the final hydroxynitrile has two alkyl groups on the carbon atom.
Due to the toxic nature of the reagent great care must be taken both in the course of the reaction and in the disposal of waste.
Reduction of aldehydes and ketones
Compounds able to provide hydride ions are able to reduce aldehydes and ketones. Suitable compounds are lithium aluminium hydride and sodium borohydride. The latter has the advantage of being able to act in aqueous solution.

Aldehydes produce primary alcohols while ketones produce secondary alcohols.
RCHO + 2[H] ![]() (NaBH4)
(NaBH4) ![]() RCH2OH
RCH2OH
RCOR + 2[H] ![]() (NaBH4)
(NaBH4) ![]() R2CHOH
R2CHOH