3.5.5 Reactions of Inorganic Compounds in Aqueous Solution - Substitution reactions
Students should:
|
Ligand exchange
This means that one ligand replaces another in a transition metal complex. Although the transition metal complex ion is stable the metal ion itself is still attracted to surrounding ligand species. If they are in a large enough concentration, or reactive enough then there is possibility of exchange.
The ease with which a transition metal loses hold of a ligand is called its lability. A labile ligand is easily lost.
In aqueous solution the number of water ligands is in huge excess and as one water ligand is indistinguishable from another, there is no overall effect of ligand exchange.
However, in ammonia solution, for example, the ammonia molecules can exchange positions with water ligands providing the ammonia is in high enough concentration. This happens with chromium(III) aqueous ions, which can exchange up to 6 water ligands with ammonia ligands.
This can be studied using colorimetry, as each species has a slighly different colour, and hence absorption, at a particular wavelength.
Coordination number
This is the number of attachments made to the central transition metal atom. The coordination number is determined by the size of the ligand particles. The larger the ligand the fewer can fit around the central metal ion.
Ammonia and water molecules are about the same size and so maintain the octahedral arrangement (coordination number = 6), regardless of ligand exchange.
Chloride ligands, however, are much larger and fit around the central transition metal ion in a tetrahedral arrangement (coordination number = 4). Hence, ligand replacement of water by chloride ions produces a change in geometry, coordination number and overall charge.
If a cobalt(II) aqueous solution has concentrated hydrochloric acid added, there is a change from a red solution to a deep blue solution:
[Co(H2O)6]2+
+ 4Cl- ![]() [CoCl4]2-
+ 6H2O
[CoCl4]2-
+ 6H2O
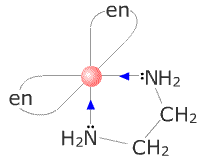
Chelating ligands
A chelating ligand is one that forms more than one attachment to the central transition metal atom/ion. Such ligands are said to be polydentate = many teeth.
Bidentate ligands include ethanediamine, H2NCH2CH2NH2
![]()
As the polydentate ligand has to organise its atoms into rings (ligand - transition metal - ligand), the ideal situation is to have unstrained rings of 5 or six bonds. Ethanediamine fulfils this requirement.