3.5.5 Reactions of Inorganic Compounds in Aqueous Solution - Lewis acids and bases
Students should:
|
Lewis acids
The Lewis theory of acids encompasses and extends the Arrhenius and Bronsted Lowry theories. According to Lewis, an acid is a species that can accept a pair of electrons. This allows treatment of non-aqueous systems in the same way as Bronsted Lowry, but also allows species that do not involve hydrogen ions.
Hence, a transition metal is classified as a Lewis acid, as it accepts lone pairs of electrons when bonding to ligands.
In the same way, all species that are involved in coordinate (dative) bonding can be classified as acids and bases.
|
Example: Aluminium chloride AlCl3 is a Lewis acid because it is electron deficient and can accept a lone pair of electrons from other species. This is seen in Friedel-Crafts catalysis:
|
Coordinate bonding
The first thing to highlight is that a coordinate bond is no different from any other bond in terms of strength, or the fact that a pair of electrons is involved in holding two nuclear centres together. The differentiation is merely a tool to explain which atom originally held the electrons used in the bond.
A normal single bond involves one electron from each atom being shared by both.
A coordinate bond involves two electrons from one atom being shared by two atoms.
 |
Transition metals are particularly good at accepting electron pairs to form complex ions. This is because the first row transition metals have available empty 4s and 4p and 4d orbitals, which can be hybridised to accept electron pairs.
Hybridisation of the 4s and 4p orbitals produces four sp3 orbitals arranged in a tetrahedral orientation:
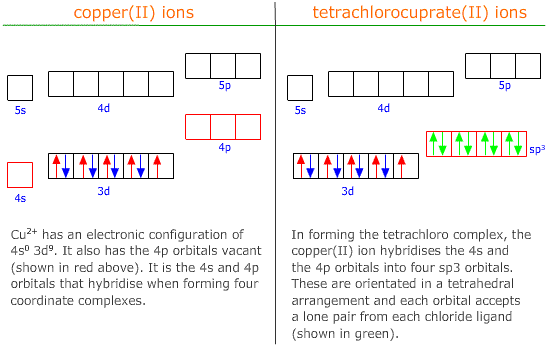 |
Hybridisation of 4s, 4p and two of the 4d orbitals creates six sp3d2 orbitals which orientate into an octahedral shape for octahedral complex formation.
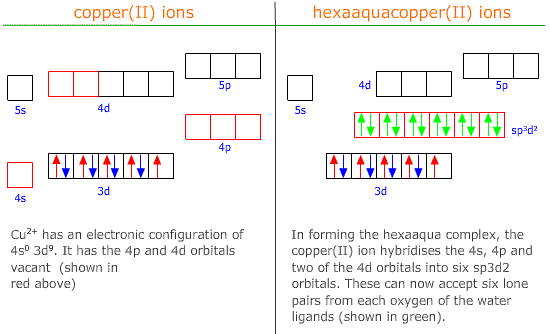 |
Ligands
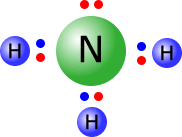
As the transition metal atoms accept pairs of electrons, then the ligands have to donate them. All ligands are Lewis bases. The diagrams below show the Lewis structures of the most common ligands with the ligand names
| aqua | hydroxy | ammine | carbonyl |
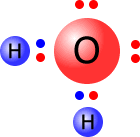 |
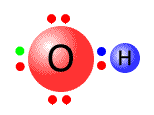 |
 |
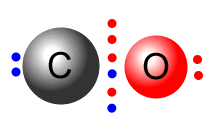 |
Notice that the carbonyl group has two lone pairs on different atoms. However, the CO group bonds using the lone pair on the carbon. Certain ligands can bond using different electron pairs, giving rise to different complexes.
For example: the cyanide ligand is isoelectronic with carbon monoxide. meaning that it has a lone pair on both the carbon and the nitrogen atoms available for coordinate bonding. When the carbon lone pair is used the ligand takes the name cyano-, but when the nitrogen atom electrons are used it becomes isocyano-.
 |
| cyano ligand (cyanide ion) |
