3.5.3 Redox Equilibria - Electrode potentials
Students should:
|
Half-equation convention
These are equations representing either the reduction or the oxidation that takes place in a redox reaction. However, as these haf equations are reversible, the convention is to always write them as reductions. In other words a species gaining electrons:
Zn2+(aq) + 2e ![]() Zn(s)
Zn(s)
Cell representation
Electrochemical cells are represented using a convention of vertical lines to indicate phase or state barriers and commas to show similar phases in contact. The salt bridge is shown as a double vertical line.
Hence the following voltaic cell:
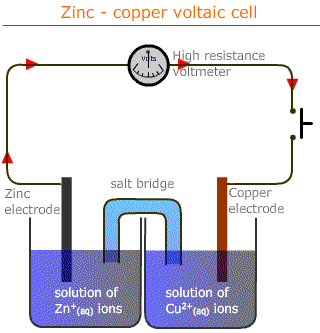
Can be represented by the following convention: Zn|Zn2+(aq)||Cu2+(aq)|Cu
The anode (where oxidation takes place) is shown at the left hand side and the cathode at the right hand side.
The standard hydrogen electrode
The position of equilibrium of an electrochemical half cell cannot be measured directly. We know that one half cel has a tendency to push electrons towards another and we can measure the potential difference between two half cells, but there is no way of knowing the actual absolute position of the equilibrium set up between a species and its reduced (or oxidised) counterpart.
The actual position of the equilibrium:
Zn2+(aq) + 2e ![]() Zn(s)
Zn(s)
cannot be measured.
So, what we do is measure the potential difference between each half cell and a standard half cell called the standard hydrogen electrode:
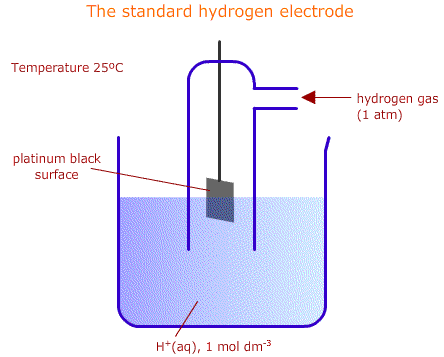
2H+(aq) + 2e ![]() H2(g)
H2(g)
The standard hydrogen electrode is defined as the reference with a value of exactly zero volts electrical potential, 0.0 V. Any other half cell can then be attached to the standard hydrogen electrode and its electrode potential recorded:

The conditions are important, because the electrode potential is affected by both concentration of the solutions and the temperature. In the case of the zinc half cell it has an electrode potential of -0.76 V. This means that compared to the standard hydrogen electrode, it forms the relatvely negative electrode (it provides electrons) by the process:
Zn(s) ![]() Zn2+(aq) + 2e
Zn2+(aq) + 2e
While the standard hydrogen elctrode is relatively more positive and undergoes the reduction reaction
2H+(aq) + 2e ![]() H2(g)
H2(g)
And the overall reaction for the zinc-standard hydrogen electrode cell: Zn|Zn2+(aq)||H+(aq)|Pt|H2(g):
Zn(s) + 2H+(aq) ![]() Zn2+(aq) + H2(g)
Zn2+(aq) + H2(g)