3.5.2 Periodicity - the acid - base properties of the oxides of Period 3 elements
Students should:
|
Properties of the period 3 oxides

Sodium forms three oxides:
- Sodium oxide, Na2O
- Sodium peroxide, Na2O2
- Sodium superoxide, NaO2
Of these three sodium oxide is considered to be the 'normal' oxide.
Physical properties
It is a white powder with a high melting point. This is indicative of its giant ionic structure.
Chemical properties
Sodium oxide is a strongly basic oxide and dissolves in water, reacting to form sodium hydroxide solution:
Na2O(s) + H2O(l) ![]() NaOH(aq)
NaOH(aq)
Sodium hydroxide is a strong alkali. 0.1M NaOH(aq) has a pH = 14.
It reacts with dilute acids forming soluble sodium salts. This is a neutralisation reaction:
Na2O(s) + HCl(aq) ![]() NaCl(aq)+ H2O
NaCl(aq)+ H2O
Magnesium forms only one oxide, magnesium oxide MgO:
Physical properties
It is a white powder with a high melting point. This is indicative of its giant ionic structure.
Chemical properties
Magnesium oxide is a weakly basic oxide and is sparingly soluble in water, in which it reacts to form magnesium hydroxide solution:
MgO(s) + H2O(l) ![]() Mg(OH)2(aq)
Mg(OH)2(aq)
Magnesium hydroxide is a weak alkali.
It reacts with dilute acids forming soluble magnesium salts. This is a neutralisation reaction:
MgO(s) + HCl(aq) ![]() MgCl2(aq)+ H2O
MgCl2(aq)+ H2O

Aluminium forms only one oxide, aluminium oxide Al2O3:
Physical properties
It is a white powder with a high melting point. This is indicative of its giant ionic structure.
Chemical properties
Aluminium oxide is an amphoteric oxide (it reacts with both acids and bases) and is insoluble in water:
Al2O3(s) + H2O(l)
![]() No reaction
No reaction
It reacts with dilute acids forming soluble aluminium salts. This is a neutralisation reaction:
Al2O3(s) + 6HCl(aq)
![]() 2AlCl3(aq)+
3H2O
2AlCl3(aq)+
3H2O
It reacts with bases to form salts in which the aluminium is in the form of a negative ion, aluminates, AlO2-.
Al2O3(s) + 2NaOH(aq)
![]() 2NaAlO2(aq)+
H2O
2NaAlO2(aq)+
H2O

Silicon forms only one oxide, silicon dioxide SiO2:
Physical properties
When pure, silicon dioxide is a white powder with a very high melting point.
Although the formula is represented with only one silicon atom and two oxygen atoms, it is actually a giant covalent molecular lattice with each silicon attached to four oxygen atoms and each oxygen atom attached to two silcon atoms. The simplest formula = SiO2.
![]()
Chemical properties
Silicon dioxide is an acidic oxide, but is insoluble in water:
SiO2(s) + H2O(l) ![]() No reaction
No reaction
It reacts with bases to form salts in which the silicon is in the form of a negative ion, silicates, SiO32-.
SiO2(s) + 2NaOH(aq) ![]() Na2SiO3(aq)+ H2O(l)
Na2SiO3(aq)+ H2O(l)

Phosphorus forms only two oxides:
- Phosphorus(III) oxide P4O6
- Phosphorus(V) oxide P4O10
The most common oxide is phosphorus(V) oxide, which is produced when phosphorus burns in a plentiful supply of air or oxygen.
Physical properties
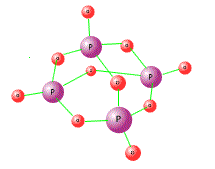
When pure, phosphorus(V) oxide is a white powder with a low melting point. The structure is simple covalent and comprises pyamids of phosphorus atoms with bridging oxygen atoms, each phosphorus surounded by a tetrahedral arrangement of four oxygen atoms:
Chemical properties
Phosphorus(V) oxide is an acidic oxide which reacts vigorously with water in a highly exothermic reaction forming phosphoric acid:
P4O10(s) + H2O(l)
![]() H3PO4(aq)
H3PO4(aq)
It reacts with bases to form phosphate salts.
P4O10(s) + 12NaOH(aq)
![]() 4Na3PO4(aq)
+ 6H2O(l)
4Na3PO4(aq)
+ 6H2O(l)

Sulfur forms only two oxides:
- Sulfur(IV) oxide, SO2
- Sulfur(VI) oxide, SO3
The most common oxide is sulfur(IV) oxide, which is produced when sulfur burns in a plentiful supply of air or oxygen.
S(s) + O2(g) ![]() SO2(aq)
SO2(aq)
Sulfur (VI) oxide is formed by the reaction between sulfur(IV) oxide and oxygen in the presence of a vanadium(V) oxide catalyst at 450ºC (the contact process)
2SO2(g) + O2(g)![]() V2O5
V2O5 ![]() 2SO3(s)
2SO3(s)
Physical properties - sulfur(IV) oxide
Sulfur(IV) oxide is a toxic gas at room temperature with a choking metallic smell. The structure is simple covalent and comprises discrete SO2 molecules. The bonding is an average of two resonance forms leaving the O-S-O bond angle = 119º, with both sulfur-oxygen bonds equal in length and energy.
 |
 |
| sulfur dioxide Lewis structure | sulfur dioxide resonance |
Chemical properties - sulfur(IV) oxide
Sulfur(IV) oxide is an acidic oxide, which dissolves readily in water forming sulfuric(IV) acid (sulfurous acid). Sulfuric(IV) acid is a weak acid:
SO2(g) + H2O(l) ![]() H2SO3(aq)
H2SO3(aq)
It reacts with bases to form sulfites (sulfate(IV) salts).
SO2(g) + 2NaOH(aq) ![]() Na2SO3(aq) + H2O(l)
Na2SO3(aq) + H2O(l)
Physical properties - sulfur(VI) oxide
Sulfur(VI) oxide is a white volatile solid at room temperature. The structure is simple covalent and comprises discrete SO3 molecules.

There are three possible resonance forms and the true structure is an average of these. The molecule is trigonal planar with bond angles of 120º having all three S-O bonds intermediate in length and strength between double and single bonds.
Chemical properties - sulfur(VI) oxide
Sulfur(IV) oxide is a very acidic oxide which dissolves exothermically in water forming sulfuric acid, a strong mineral acid. The reaction produces so much heat energy that it is not usually carried out, as it results in a dangerous mist of sulfuric acid and water vapour.
SO2(g) + H2O(l) ![]() H2SO4(l)
H2SO4(l)
To prepare sulfuric acid in industry, sulfur trioxide from the contact process is dissolved in 98% sulfuric acid making 100% sulfuric acid. water can be carefully added to this to make more 98% sulfuric acid, etc.
SO3(g) + 98%H2SO4(aq)
![]() 100%H2SO4(l)
100%H2SO4(l)
H2SO4(l) + H2O(l)
![]() 98%H2SO4(aq)
98%H2SO4(aq)
Sulfur trioxide reacts with bases to form sulfates (sulfate(VI) salts).
SO3(g) + 2NaOH(aq) ![]() Na2SO4(aq) + H2O(l)
Na2SO4(aq) + H2O(l)
Summary
The acid-base character of the period 3 oxides changes from left to right from basic to amphoteric to acidic. This is in line with the change in structure and nature of the elements from reactive metals to poor metals to non-metals.
The oxides increase in acidity with increasing oxidation state. Hence, sulfur(IV) oxide is less acidic than sulfur(VI) oxide. This is in line with the increase tendency towards covalent character in higher oxidation states.
In general: Left to right in the periodic table = decreased metallic character = increased covalent character = increasing acidity of oxides.