3.2.10 Alcohols - Elimination
Students should:
|
Elimination reactions
Dehydration to form alkenes or alkoxyalkanes
The products formed depend on the conditions used: Alkenes are formed in the presence of H2SO4 (or H3PO4 better, as it doesn't produce as many by-products) and the correct temperature (hot for primary, warm for secondary and cool for tertiary) alcohols lose a water molecule.
|
Example: Dehydration of ethanol CH2H-CH2OH |
The temperature for primary alcohols must be kept above 170ºC to prevent formation of ethers.
The alcohol group is eliminated along with hydrogen atom from the carbon adjacent to the carbon holding the alcohol group. If there is no hydrogen on this carbon then elimination is not possible.
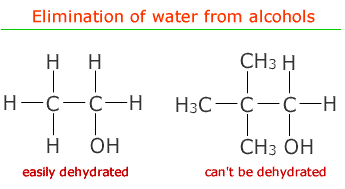
One of the advantages of this method of preparing alkenes is that the starting materials, the alcohols, can be produced by biological means such as fermentation. This means that plastic manufacture does not have to depend on the petrochemicals industry.
Fermentation  alcohols
alcohols  dehydration
dehydration  alkenes
alkenes  polymerisation
polymerisation  polymers
polymers