3.2.9 Alkenes - Addition reactions
Students should:
|
Electrophilic addition of alkenes
Alkenes have an electron rich double bond with one of the pairs of electrons in an easily broken pi orbital. This acts as a region of attraction for positive or partially positive particles (electrophiles).
These electrophiles can cause the double bond to 'open', allowing other atoms or groups to be added to the original carbon atoms of the double bond. This is called addition.
Overall, the reaction is called electrophilic (attack by an electrophile) addition (the overall result).
Hydrogen bromide
The hydrogen bromide molecule is polarised as bromine has a higher electonegativity than hydrogen. The reaction with an alkene takes place in two steps.
- 1 Attack by the electrophile opening the double bond and producing an intermediate carbonium ion and leaving a negative bromide ion
- 2 The bromide ion then joins to the positive carbon atom of the carbo-cation (carbonium ion)

Sulfuric acid
Sulfuric acid has two hydrogen atoms that are easly lost (that is why it is acidic). This allows it to behave as if it were HSO3O- and H+.
The hydrogen ion behaves as an electrophile adn the reaction proceeds in the same way as for hydrogen bromide.

Bromine
The bromine molecule is not polarised so it seems at first sight that it should not cause electrophilic addition of alkenes. However, the approach of the bromine molecule to the electron rich double bond induces temporary polarisation within the bromine molecule.
- 1 The bromine molecule approaches the alkene double bond and becomes polarised by the repulsive effect of the region of negative charge
- 2 The polarised molecule can then attack the double bond
- 3 An intermediate carbo-cation and a bromide ion is formed
- 4 The bromide ion adds to the positive carbon atom

This reaction is used as a test for alkenes. The product (1,2-dibromoethane in this case) is colourless, while bromine is either a dark red liquid in pure form or an orange solution when dissolved in water.
Hence the test for unsaturation (double bonds) is to add bromine water and if it becomes decolourised it indicates the presence of one or more double bonds.
Asymmetric electrophilic addition
Electrophilic addition of hydrogen bromide (or any other asymmetric electrophile) to asymmetrical alkenes gives a choice of two possible products.

Markovnikov's rule says that in the case of asymmetric electrophilic addition, the hydrogen adds to the carbon atom that already has more hydrogen atoms attached. In the case above the hydrogen atom fromm the hydrogen bromide would add to the left hand side carbon, i.e. route 1 would be preferentially followed.
This does not exclude the possibility of route 2, it's just that this would be a minor product (perhaps only a couple of percent).
Why does Markovnikov's rule apply?
The key to understanding Markovinikov's rule is the stability of the intermediate formed in the two possible routes of the mechanism. In both routes the intermediate is a carbonium ion. In route 2 the intermediate carbonium ion positive charge resides on a carbon which is attached to two hydrogen atoms. This does nothing to help stabilise the ion.
However, in route 1 the carbonium ion positive charge resides on a carbon which has an alkyl (R) group attached. Alkyl groups can be considered to act as little 'electron pumps' and induce negative charge away from themselves. This induction of negative charge stabilises the positive charge on the carbonium ion by reducing it and makes the ion more stable.
A more stable ion is more easily formed and so the mechanism for the addition preferentially proceeds via this stable ion. Route 1 is favoured.

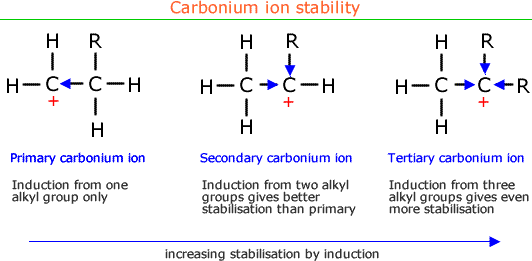
It should be appreciated at this point that the presence of two alkyl groups on a carbon atom will be ale to stabilise an intermediate even more. This gives risae to a stabilisation order for carbocations (carbonium ions).
Tertiary carbonium ions are more stable than secondary carbonium ions, which are more stable than primary carbonium ions.
Manufacture of alcohols
Alkenes can be persuaded to add water in the presence of a catalyst in a reaction very similar to the electrophilic addtion reactions above. The conditions need to be adjusted and the alkene mixed with steam and passed over a phosphoric acid catalyst at 300ºC and 60-70 atmospheres pressure. The reaction between ethene and steam only proceeds to equilibrium with a small quantity of ethanol formed, but by recycling the unreacted gases the conversion can be taken to 100%.
CH2=CH2 + H2O
![]() CH3CH2OH
CH3CH2OH
When the alkene is ethene then the product is ethanol. This is a very useful way of manufacturing ethanol from the ethene produced in the petrochemicals industry.
Other industrially important alcohols can also be manufactured by this process.