3.2.8 Haloalkanes - Elimination
Students should:
|
Elimination of haloalkanes
Haloalkanes react with a solution of sodium hydroxide in ethanol by eliminating a molecule of water and forming a double bond. They can only do this if there is a hydrogen atom on the carbon atom adjacent to the atom that holds the halogen.
|
1-chloropropane + sodium hydroxide/ethanol ClCH2CH2CH3 +
NaOH/ethanol |
 |
 |
|
1-chloropropane
(can react) |
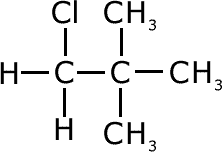
chlorodimethylpropane
(cannot react) |
Mechanism of elimination reactions
Once again the mechanism depends on the nature of the haloalkane. Primary haloalkanes react via an E2 mechanism and tertiary haloalkanes via an E1 mechanism. Secondary mechanisms are probably a combination of both E1 and E2.
- E1 = Unimolecular elimination
- E2 = Bimolecular elimination
In bimolecular elimination the first stage involves two colliding particles. Firstly, the strong base produced by the solution of sodium hydroxide in ethanol (the ethoxide ion) removes a hydrogen atom from the carbon that is adjacent to the carbon atom holding the halogen.
|
2-bromopropane + ethoxide ion 2-bromopropane carbanion + ethanol CH3CHBrCH3 + EtO |
Then the carbanion formed makes a double bond, ejecting the halide ion in the process:
|
2-bromopropane |
- Step 1: Abstraction of a proton by the ethoxide ion.
- Step 2: Electron pair goes between the two carbon atoms making a double bond and at the same time dislodging the bromide ion.
Overall: Hydrogen bromide eliminated and alkene formed.
In E1 elimination, the halogen breaks off the tertiary haloakane before the hydrogen ion is collected by the ethoxide ion (base).