3.1.5 Introduction to Organic Chemistry - Isomerism
Students should:
|
Isomerism and branching
Any structure that can be drawn can exist providing the fundamental rules have been fulfilled.
- 1 Each carbon forms four bonds (may be all single, one double and two singles, etc)
- 2 Each hydrogen forms one bond
- 3 Each oxygen forms two bonds
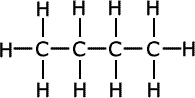
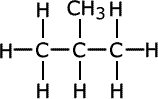
This means that one molecular formula can have other possible structures. This is called isomerism. The chains formed by the alkanes and other organic molecules do not have to be straight (actually zigzag) but may be branched, i.e. having "branches" of carbon atoms attached to the main unbroken chain.
This particular form of isomerism is called chain isomerism.
|
Example: The alkane C4H10 exists in two isomeric forms - a straight chain form and a branched form
|
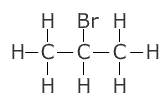
Positional isomerism
This is when atoms, or groups attached to a hydrocarbon chain change their position:
| 1-bromopropane | 2-bromopropane |
 |
 |
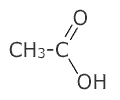
Functional group isomerism
This is when the atoms of a structure are rearranged to such an extent that the actual functional group changes:
| ethanoic acid | ethene-1,2-diol |
 |
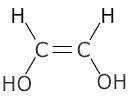 |
The molecular formula of both structures above is C2H4O2, but it is clear that they are completely different molecules, one a carboxylic acid and the other an unsaturated (double bond) diol (two alcohol groups).